
Evusheld drug approved for use in people aged 12 and older who are immunocompromised or for whom COVID-19 vaccination is not recommended.
Health Canada has authorized a drug developed by AstraZeneca to prevent COVID-19 infections.
Evusheld has been cleared for people aged 12 and older who have a compromised immune system and are unlikely to get an adequate response to a COVID-19 vaccination, or for whom COVID-19 vaccination is not recommended.
The medication contains lab-made antibodies designed to remain in the body for months, which officials say will contain the virus if there is an infection.
The therapy has also been authorized in the United States and has been recommended by the European Medicines Agency.


 Welland CAO Optimistic for 2026
Welland CAO Optimistic for 2026
 Niagara Clinics Help Coughs, Colds
Niagara Clinics Help Coughs, Colds
 Clifton Hill Construction in January
Clifton Hill Construction in January
 Gas Prices to Hold Steady: Analyst
Gas Prices to Hold Steady: Analyst
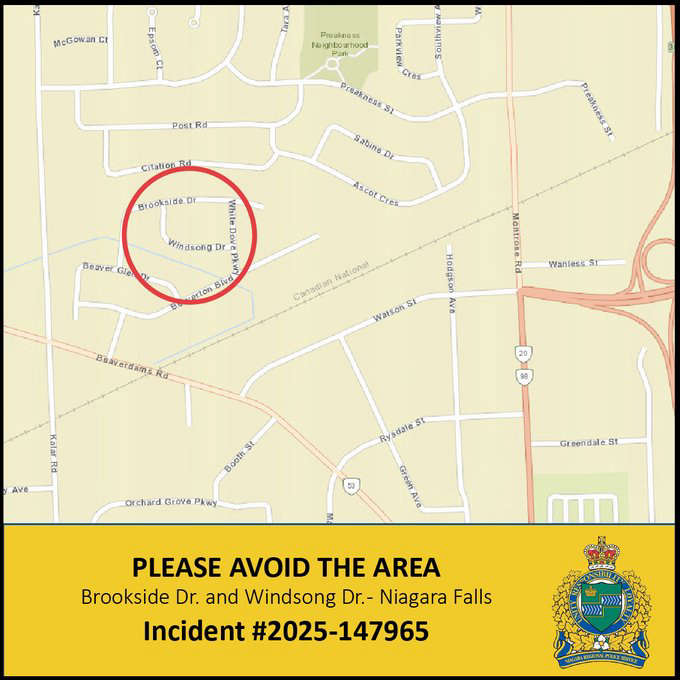 NRP Using Warrant in Niagara Falls Area
NRP Using Warrant in Niagara Falls Area
 Part of Welland Canal Officially Named
Part of Welland Canal Officially Named
 Girl Assaulted in Break and Enter
Girl Assaulted in Break and Enter
 Invest in Officer Well-Being: Former Chair
Invest in Officer Well-Being: Former Chair